Clinical Trials &
Managed Access Program
Lonafarnib Managed Access Program: Another pathway to global access
In August 2019, Eiger BioPharmaceuticals, manufacturers of lonafarnib, launched the lonafarnib Managed Access Program (MAP). MAP allows eligible children and young adults with Progeria and Progeroid Laminopathies to obtain lonafarnib through their local physicians in countries that allow MAP to be offered.
For doctors to enroll their patients in this program, please email Clinigen’s Medicine Access team at medicineaccess@clinigengroup.com or call +44 (0) 1932 824123.
Progeria Clinical Drug Trials: Background
 Progeria clinical drug trials are the best hope for children with Progeria, testing potential treatments that may enable them to live longer, healthier lives. These trials are the culmination of years of research focused on what drug or combination of drugs will treat and cure the children.
Progeria clinical drug trials are the best hope for children with Progeria, testing potential treatments that may enable them to live longer, healthier lives. These trials are the culmination of years of research focused on what drug or combination of drugs will treat and cure the children.
Since 1999 when we founded PRF and there were no resources for these children, we have soared from total obscurity, to gene finding, to the first Progeria clinical trials, to the first-ever FDA-approved treatment, called lonafarnib – all at a pace virtually unheard of in the scientific community. And while helping this handful of children, Progeria’s connection to common heart disease and aging has tremendous implications for us all.
What’s Next for Treatments and Cure?

Kickstarting a New Clinical Drug Trial: Preparations for Progerinin Trial Have Begun!
PRF, in collaboration with Korean-based study sponsor PRG Science & Technology (PRG S&T), is hoping to kick off a brand-new clinical trial soon with a drug called Progerinin. Laboratory evidence shows that this drug, when taken in combination with lonafarnib, may be more effective than lonafarnib alone. PRF funded the laboratory work that led to the formation of PRG S&T and its development of Progerinin. Pre-trial work has begun at Boston Children’s Hospital, in anticipation of bringing children from all over the world to enroll for this trial in the coming months. We’re super excited about starting a new trial with such a promising drug, and look forward to sharing more details with you as they become available.
PRF co-founder and Executive Director Audrey Gordon seals the deal in June with Dr. Bum-Joon Park of PRG S&T to move forward with pre-trial work.
RNA Therapy: Drug Administration Feasibility Study Has Begun!
PRF has taken the first patient-involved steps toward a clinical trial in RNA therapy – SO exciting!
Background: In January 2021, we reported breakthrough findings in RNA therapeutics, wherein this therapy inhibited production of RNA coding for the Progeria disease-causing protein, progerin. The study*, led by Dr. Francis Collins, White House Science Advisor and former Director of the National Institutes of Health (NIH), revealed that Progeria mice treated with a drug named SRP-2001 reduced the harmful progerin mRNA and protein expression in blood vessels, as well as in other tissues. The blood vessels were stronger, and the mice showed an increased survival of over 60% compared to untreated mice. Thus the work with this promising therapy continued, and we are taking the next step with a Feasibility Study as follows:
Typically, RNA therapeutics are liquids that are injected intravenously (directly into the vein). However, those with Progeria would not be able to tolerate intravenous delivery of the required daily dosage. Thus, PRF developed a subcutaneous delivery system whereby the liquid can be injected with a small needle under the skin. A 6-month study is now underway at BCH to determine the feasibility of this delivery approach for those with Progeria. The team is testing whether administration of a saline solution can comfortably be injected subcutaneously. If it is successful, we will be one step closer to a clinical trial in genetic therapy!
*Erdos, M.R., Cabral, W.A., Tavarez, U.L. et al. A targeted antisense therapeutic approach for Hutchinson–Gilford progeria syndrome. Nat Med (2021).
What’s Happening TODAY With the Progeria Clinical Trials?
The most recent trial involved 2 drugs: lonafarnib and a new drug, everolimus. Phase 1, to determine the safe and appropriate dosage of everolimus, began in April 2016 and was successfully completed in June 2017. Phase 2, which tested the effectiveness of the 2-drug combination, began in July 2017 and was completed in April 2022. Sixty children from 27 countries were enrolled in this two-drug phase!
We have now entered a period of data analysis and expect to eventually publish results in a peer-reviewed, scientific journal. In the meantime, trial participants have either rolled over into the monotherapy extension of the trial, or into Eiger’s Managed Access Program. Through either pathway, participants continue to be supplied with lonafarnib, the current standard of care.
Everolimus is a form of the drug rapamycin; everolimus could be more easily given to children with Progeria because it requires fewer blood draws to measure drug levels. While lonafarnib may block the toxic progerin from developing, rapamycin appears to allow cells to more rapidly clear out progerin. Thus with rapamycin targeting a different pathway than lonafarnib, the combination may prove to be a “one-two punch” to Progeria – hopefully a better treatment than lonafarnib on its own.
Trial History at a Glance
To date, PRF has funded and co-coordinated three clinical trials (two of which had two parts, phases 1 and 2). PRF is and always has been responsible for all trial expenses, including testing, travel, food, lodging, translators and staff. Each new trial is more expensive than the last, as more children enroll for a chance at longer, healthier lives.
Details on the Prior Trials
#1 involved a single drug, lonafarnib, began in 2007, and proved successful. Read all about the historic treatment discovery here.
#2, the “Triple Trial” involved 3 drugs: lonafarnib, pravastatin and zoledronate. This began with a 1-month, phase 1 “mini trial” in March 2009 to determine if adding 2 more drugs to the lonafarnib regimen was safe to move forward with a larger population (which it was). Phase 2 began in August 2009. Its protocol changed over the course of five years, switching back to just lonafarnib and re-opening enrollment so more children could participate. Read more here.
#3 is the two-drug, lonafarnib and everolimus trial. Phase 1, to determine the safe and appropriate dosage of everolimus, began in April 2016 and was successfully completed in June 2017. Phase 2, which tested the effectiveness of the 2-drug combination, began in July 2017 and was completed in April 2022. The monotherapy extension of this trial continues today.
May 7, 2007: The Start of the First-Ever Progeria Clinical Drug Trial Marks Historic Moment in Progeria Research History!
In 2006, researchers identified a potential drug treatment for children with Progeria, called FTIs. For the first time, we had in front of us a possible treatment for children with Progeria. Exciting times! The Progeria clinical drug trial began on May 7th, 2007 with two children – Meghan and Megan – arriving at Boston Children’s Hospital in Boston, MA for their first of seven visits over a 2-year period. At this first visit, they were given extensive tests and their first doses of the drug. An average of two families traveled to Boston each week thereafter, through December 2009, followed by a period of time in which the trial team analyzed the many thousands of data elements (each child underwent over 100 tests per visit!) and sought publication of the results.
“I know of no other rare genetic disease that has gone from gene discovery to clinical trial in under four years – a phenomenal testament to the hard work of The Progeria Research Foundation.”
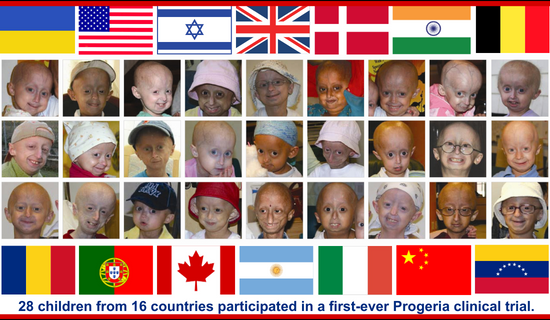
Twenty-eight (28) children from sixteen countries participated, ages 3 to 15 years. Children returned to Children’s Hospital Boston every four months, for testing and to receive new drug supply, and stayed in Boston for 4-8 days each visit. While at home, their doctors kept a close watch over the children and submitted periodic health reports to the Boston research team. For the duration of the trial, an average of 2 children per week traveled to Boston to participate.
Who, Where, When, How and How Much…
The first three clinical trials were led by Mark Kieran MD, PhD, Director, Pediatric Medical Neuro-Oncology, Dana-Farber Cancer Institute and Children’s Hospital Boston; Assistant Professor, Departments of Pediatrics and Hematology/Oncology, Harvard Medical School. Dr. Kieran is a pediatric oncologist with extensive experience with the drug under study (farnesyltransferase, or FTI) in children. In 2017, he left his position at Dana Farber to work in the private sector. Co-Chairs were Monica Kleinman, MD, Director of Medical-Surgical Intensive Care Unit, Sr. Associate in Critical Care Medicine at BCH, Assistant Prof. at Harvard Medical School; and Leslie Gordon, MD, PhD, Medical Director of PRF, Lecturer at BCH and Harvard Medical School, Professor of Pediatrics at Hasbro Children’s Hospital and Brown University in Providence, RI. Dr. Kleinman has assumed the lead role of Principal Investigator.
The clinical trials are a collaborative effort, involving physicians at Boston Children’s Hospital, Dana-Farber Cancer Institute, and Brigham and Women’s Hospital, all Harvard University institutions. In addition, physicians and scientists from The Warren Alpert Medical School at Brown University and NIH helped to make this first and the other trials a success.
How did we get to this point?
In 2003, The Progeria Research Foundation’s collaborative research team discovered the Progeria gene. This discovery not only led to further understanding of Progeria, but scientists now know that studying Progeria can help us learn more about heart disease and the normal aging process that affects us all. Since the gene discovery, the support of researchers, clinicians, families of children with Progeria and people like YOU brought us to another crossroads in the search for a treatment. Researchers began an intense study of this enemy protein called progerin, and in 2006 they identified a potential drug treatment for children with Progeria, called farnesyltransferase inhibitors (FTIs), and conducted studies in the lab that supported a human trial with the drug. The FTI chosen was initially supplied by Merck and called lonafarnib. Click here for more details on the research.
Why did researchers think this drug would work in Progeria?
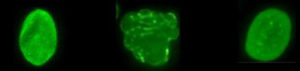
Normal cell, Progeria cell, Progeria cell after being treated with FTI.
The protein that we believe is responsible for Progeria is called progerin. In order to block normal cell function and cause Progeria, a molecule called a “farnesyl group” must be attached to the progerin protein. FTIs act by blocking (inhibiting) the attachment of the farnesyl group onto progerin. So if the FTI drug can block this farnesyl group attachment in children with Progeria, then progerin may be “paralyzed” and Progeria improved. Click here for more information on FTIs.
How did PRF fund the trial?
Thanks to the support of thousands, we were able to raise all the funds necessary to cover the trial costs. Our heartfelt gratitude goes out to everyone who contributed their “time, talents and treasure” to make this incredible achievement possible, and of course to all the courageous families who participated.
The FTI lonafarnib is now a proven treatment for Progeria.
In 2012, the study results were published, demonstrating that every child experienced improvement in one or more areas, including the vital cardiovascular system. In May, 2014, a study showed one or more of 3 drugs – including lonafarnib – being tested in PRF-funded clinical trials extended lifespan; it was unclear which drug had this positive life-changing impact. However, in April 2018, a study published in The Journal of the American Medical Association (JAMA) reported that lonafarnib alone extended survival in children with Progeria by at least 1.6 years. Click here for details on the 2012 historic treatment discovery study, here for details on the 2014 findings, and here for details on the 2018 study.
“EVERYONE has been so wonderful. To us you are ALL GOD SENT and we APPRECIATE all that you do for these little angels. Our family is so overwhelmed with excitement and all sorts of emotions with Adalia’s trip to Boston this weekend, I can’t even begin to type the words of how we are feeling.”
“This new medication for Zach gives us a renewed hope that his heart will be stronger, his smile will be brighter and his life will be longer. This new drug trial is an answer to our prayers. Thank you to everyone involved with PRF who made this happen…the doctors, the researchers and the staff. You are our heroes!”
“On behalf of Cam and our family, thank you all at PRF so much for all you have done! We would have been lost in a world of confusion and grief without you. Instead, we live in a world of hope and purpose. Thank you again and again! With much love and respect.”

Always Moving Forward: The Progeria Triple Drug Trial Begins August 2009
Summary:
Researchers identified two additional drugs that, when used in combination with the current FTI drug being tested (lonafarnib), may provide an even more effective treatment for children with Progeria than FTI’s alone. Pravastatin and zoledronate were added to the current treatment lonafarnib. This much larger trial included 45 children from 24 different countries!
Strategy:
All three drugs target different points along the pathway leading to production of the disease-causing progerin. In exciting laboratory studies presented by Dr. Carlos Lopez-Otin of Spain at the 2007 Progeria Research Foundation Scientific Workshop, the two new drugs improved disease in Progeria cells and extended lifespan in mouse models of Progeria.
Goal:
If the three drugs administered in this trial can effectively block this farnesyl group attachment, then progerin may be “paralyzed” and Progeria may be improved even more than it is with the lonafarnib alone. The hope is that the drugs would work as partners, to complement each other so that the progerin protein is affected more by combining the three drugs.
The Feasibility Trial:
The team conducted a mini-trial for 5 children with Progeria. The short, one-month “feasibility” trial asked whether the three-drug combination would be well-tolerated, prior to embarking on a larger international trial. Side effects were acceptable, and the team moved ahead to the larger efficacy trial.
The Efficacy Trial:
45 children enrolled in this trial, from 24 countries, speaking 17 languages. This includes children that participated in the FTI-only trial, the participants in the feasibility trial, and other children that were either too young to participate in the first trial or children that we discovered during the first clinical trial (after enrollment had ended). Children enrolled in the FTI-only trial had the opportunity to enroll in the triple trial when they participated in their last visit for the current trial. This allowed those children to continue taking FTI without any missed doses.
Trial Medications at a Glance
Pravastatin (marketed as Pravachol or Selektine) is a member of the drug class of statins. It is usually used for lowering cholesterol and preventing cardiovascular disease.
Zoledronic acid is a bisphosphonate, usually used as a bone drug for improving osteoporosis, and to prevent skeletal fractures in people suffering from some forms of cancer.
Lonafarnib is an FTI (Farnesyltransferase inhibitor), a drug that can reverse an abnormality in Progeria cells in the laboratory, and has improved disease in Progeria mice.
All 3 drugs block the production of the farnesyl molecule that is needed for progerin to create disease in Progeria.
* “Combined treatment with statins and aminobisphosphonates extends longevity in a mouse model of human premature aging”, by Ignacio Varela, Sandrine Pereira, Alejandro P. Ugalde, Claire L. Navarro, Marıa F. Suarez, Pierre Cau, Juan Cadinanos, Fernando G. Osorio, Nicolas Foray, Juan Cobo, Felix de Carlos, Nicolas Levy, Jose MP Freije and Carlos Lopez-Otın. Nature Medicine, 2008. 14(7): p. 767-72.
In July a study ** was published that showed no significant improvements were found over and above the lonafarnib single therapy. **Gordon, et. al., Clinical Trial of Protein Farnesylation Inhibitors lonafarnib, Pravastatin and Zoledronic Acid in Children with Hutchinson-Gilford Progeria Syndrome, Circulation, 10.1161/CIRCULATIONAHA.116.022188
However, the “Triple Trial” was extended beyond its original 2-3-year timeframe, and expanded to include up to 80 children, so that every child could have access to lonafarnib alone because we know that it is helping the children. Usually, clinical trials run their course and the patients are taken off all the drugs until FDA approval; this could take years. PRF has ensured that the children continue to take the one known treatment, while they and their research partners continue exploring additional treatment options (such as everolimus that is currently being tested).
The Addition of a Newer Drug: Everolimus
Everolimus is a form of the drug rapamycin; everolimus could be more easily given to children with Progeria because it requires fewer blood draws to measure drug levels. While lonafarnib may block the toxic progerin from developing, rapamycin appears to allow cells to more rapidly clear out progerin. Thus with rapamycin targeting a different pathway than lonafarnib, the combination may prove to be a “one-two punch” to Progeria – hopefully a better treatment than lonafarnib on its own.
The Science Behind the Addition of this Second Drug
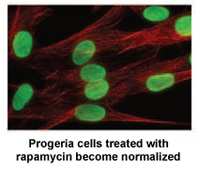
Rapamycin is an FDA-approved drug that has previously been shown to extend the lives of non-Progeria mouse models. A study* by researchers at the NIH in Bethesda, MD and Massachusetts General Hospital in Boston demonstrates that rapamycin decreases the amount of the disease-causing protein progerin by 50%, improves the abnormal nuclear shape, and extends the lifespan of Progeria cells in the laboratory.
Rapamycin is known for its anti-aging properties in mice. These findings are part of a growing list of studies that help to validate the theory that finding the cure for Progeria may also benefit the entire aging population.
* K. Cao, J. J. Graziotto, C. D. Blair, J. R. Mazzulli, M. R. Erdos, D. Krainc, F. S. Collins, “Rapamycin Reverses Cellular Phenotypes and Enhances Mutant Protein Clearance in Hutchinson-Gilford Progeria Syndrome Cells.” Sci. Transl. Med. 3, 89ra58 (2011).
The Progeria Research Foundation provided cells for this project from the PRF Cell & Tissue Bank and helped fund the research through our grants program – more proof that PRF’s research-related programs are essential to advancements toward the cure.
This 2-drug trial has been a collaborative effort that built upon the knowledge gained from PRF’s previous clinical trials. The children were seen by virtually the same team of physicians from Boston Children’s Hospital and Brigham and Women’s Hospital, all of whom now have world-renowned expertise in Progeria as well as in the drugs involved.
Sixty children from 27 countries were enrolled in this two-drug phase. Data from the 2-drug portion of the trial is being analyzed and results are being formulated and written up for publication in a peer reviewed scientific journal.
Our quest for the cure continues…
Our work with genetic therapies is advancing full-speed ahead! RNA therapy and DNA Gene Editing studies have shown a vast improvement in Progeria mice lifespan. PRF continues to invest substantial funds into their development, with the hope that these research efforts will lead to clinical trials, and, ultimately, the cure.
These cutting-edge therapies have huge potential! With your help, PRF can continue to move forward as quickly as possible toward the most effective treatments and the cure.

