History has been made, with every child in the first-ever Progeria clinical drug trial showing improvement in one or more areas of their condition, proving that the FTI drug lonafarnib is the first known, effective treatment for children with Progeria.
Click here for your free copy of the clinical trial results paper
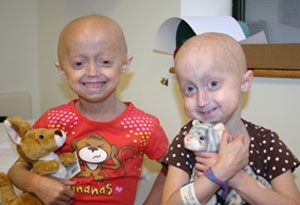
Megan and Meghan were the first two children to enroll in the trial. The two girls met in Boston every four months for two years, for testing, to receive new drug supply, and to play together! Here they are in December 2008, on a break from tests at Boston Children’s’ Hospital.
FOR MEDIA: click here for the press release, b-roll and other press details.
The results of the first-ever clinical drug trial for children with Progeria are in and it’s official! Lonafarnib, a type of farnesyltransferase inhibitor (FTI) originally developed to treat cancer, has proven effective for Progeria. Every child showing improvement in one or more of four ways: gaining additional weight, better hearing, improved bone structure and/or, most importantly, increased flexibility of blood vessels. Results of the study, which was funded and coordinated by The Progeria Research Foundation, were published September 24, 2012 in Proceedings of the National Academy of Sciences.
Gordon et. al., Clinical Trial of a Farnesyltransferase Inhibitor in Children with Hutchinson-Gilford Progeria Syndrome, PNAS, October 9, 2012 vol. 109 no. 41 16666-16671

Natsuki, from Japan, with her brother, father and mother who prepares the lonafarnib-liquid sweetener mixture.
Results Yield Improvements in All Children
Twenty-eight children from sixteen countries participated in the 2 ½ year drug trial, representing 75 percent of known Progeria cases worldwide at the time the trial began. The children traveled to Boston every four months to receive comprehensive medical testing and study medications through Boston Children’s Hospital’s Clinical and Translational Study Unit. All received oral lonafarnib, an FTI supplied by Merck & Co., twice-a-day for two years, under the supervision of clinical trial chair Mark Kieran, M.D., Ph.D., Director of Pediatric Medical Neuro-oncology at the Dana-Farber / Children’s Hospital Cancer Center, and co-chairs Dr. Monica Kleinman and Dr. Leslie Gordon
Rate of weight gain was the primary outcome measure, because children with Progeria experience severe failure to thrive, with a very slow linear rate of weight gain over time. The researchers examined many other areas of the body, including arterial stiffness (a predictor of heart attack and stroke), bone rigidity (an indicator of bone strength) and hearing. “When we started this clinical trial we had no idea whether any aspect of Progeria would be reversible, because no one had ever conducted a clinical treatment trial for Progeria before. We discovered that, among other things, the major blood vessels can actually improve. This was a breakthrough discovery for the children, since accelerated cardiovascular disease is the cause of death in Progeria. Though there is no way to know whether we have delayed strokes, heart attacks, or increased longevity within just a 2-year treatment period, these positive results compel us to continue pushing for new treatments until we accomplish what we set out to do in 1999. We want children with Progeria to live until they’re 80 and beyond. We want them to live full, healthy lives,” said Dr. Gordon, PRF’s Medical Director and first author of the treatment discovery paper.
Record Pace of Progress
The treatment discovery comes less than a decade after PRF and now National Institutes of Health Director Dr. Francis Collins joined forces to identify the cause of Progeria – an unheard of timeline in the world of medical research! But for PRF and children with Progeria, who live to an average age of just 13 years, such speed is vital to win this race against time.
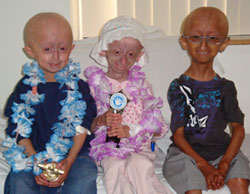
Mateo, Milagros and Fernando are all smiles upon receiving awards from PRF at their final visit. The trophies say, “YOU DID IT! You finished the 1st Progeria Trial – You’re A SUPERSTAR!”
“PRF is a good example of an organization successfully enabling translational research, moving from gene discovery to clinical treatment at an unprecedented pace,” said Dr. Kieran. “From 1999, when the organization was founded, to today, PRF has identified the genetic mutation that causes the disease, funded preclinical research, completed this trial, initiated a second trial, and is currently working with our team at Boston Children’s Hospital to plan yet another trial with drugs that, like FTIs, have shown exciting results in Progeria cells and animal models. That’s an awesome track record of accomplishment.
WE AGREE! How Did We Get to This Wonderful Day?
Following the 2003 discovery of the gene mutation that causes Progeria, PRF-funded researchers identified FTIs as a potential drug treatment. The Progeria-causing mutation leads to the production of the protein progerin, which damages cell function. Part of progerin’s toxic effect on the body is caused by a molecule called a “farnesyl group,” which attaches to the progerin protein and helps it damage the body’s cells. FTIs act by blocking the attachment of the farnesyl group onto progerin, reducing the harm progerin causes.
For more study details, CLICK HERE for the press release
Progeria Linked to Normal Aging Process
Research shows that the Progeria-causing protein progerin is also produced in the general population and increases with age. Researchers plan to continue exploring the effect of FTIs, which may help scientists learn more about the cardiovascular disease that affects millions, as well as the normal aging process that affects us all.
“Connecting this rare disease and normal aging is bearing fruit in an important way…valuable biological insights are gained by studying rare disorders such as Progeria. Our sense from the start was that Progeria had a lot to teach us about the normal aging process.”
– Dr. Francis Collins, Director of the National Institutes of Health

What a smile! Maria really enjoyed painting during one of her breaks from testing at Boston Children’s Hospital.
Help Us Find All Children With Progeria so They, Too, Can Benefit From Our Work
Researchers believe that at any given time, there are 200-250 children living with Progeria. To identify unknown children, PRF launched the “Find the Other 150” campaign in October 2009, and as of September 2012, we know of 96 children living in 35 countries- an 83% increase!! You can help find more so they can benefit from the unique treatment and care that PRF provides. These new children may be eligible for future clinical trials, so please go to Find the other 150 to find out how you can help make that happen.
Thank you ALL – We Couldn’t Have Done it Without You!
One of the main reasons we achieved breakthrough results in this first trial is because of the tremendous supporters who provided funding and other support, helping to get us one step closer to achieving our ultimate goal – a cure for Progeria. Click here to see a special tribute to all those who helped make the dream of a treatment a reality.
