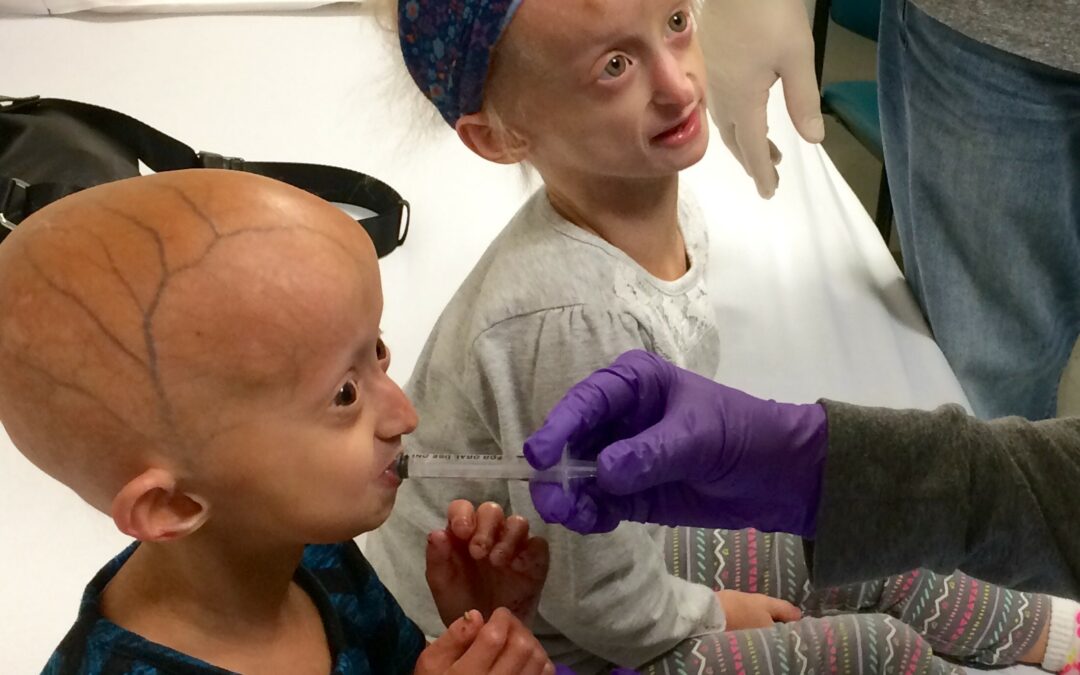
May 30, 2020 | News, Uncategorized
Thank YOU! Through these uncertain times, one thing is certain: The Progeria Research Foundation is still working tirelessly to help families impacted by Progeria. PRF is there for the children and their families from day one, so for this year’s ONEpossible Campaign,...

Apr 1, 2020 | News, Uncategorized
First and foremost, we hope you are staying well. In light of the recent progression of COVID-19, and as we all navigate this uncertain time, we want to THANK YOU for your support and let you know that our fight against Progeria remains steadfast: PRF staff continue...

Sep 19, 2019 | News, Uncategorized
Due to the incredible success of previous years’ campaigns in 2009 and 2015, we are thrilled to announce the 2019 launch of our ‘Find the Children’ initiative to search globally for the undiagnosed children with Progeria so that they, too, can have access to the...